An adverse event is defined as an event that could have caused, or did result in, harm to people including death, disability, injury, disease or suffering and/or immediate or delayed emotional reaction or psychological harm [HIS].
Health services in Scotland aim to provide high quality care that is safe, effective and person-centred. When an adverse event occurs, the focus must be on learning from what happened and increasing the safety of the healthcare system.
Incidents identified by Community Pharmacy
Community pharmacy contractors should have internal processes for reporting and investigating adverse events. Incidents involving controlled drugs must also be reported to the CDAO and an incident report completed. More information on reporting CD incidents can be found on the Controlled Drugs Governance Team webpage.
Incidents identified by NHS Lothian
Where an adverse event relating to community pharmacies is identified by NHS Lothian staff, the following process will be followed:
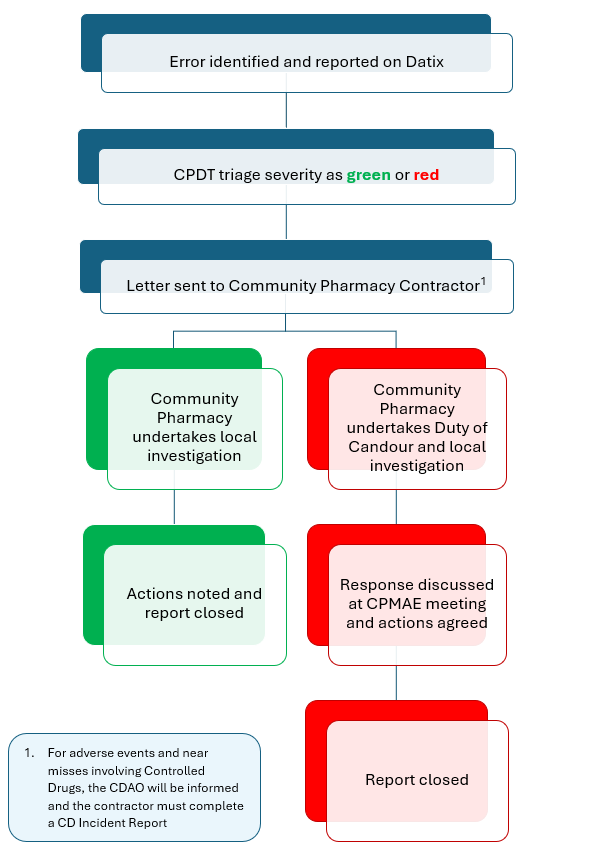
CPDT = Community Pharmacy Development Team
CPMAE = Community Pharmacy Medication Adverse Event
CDAO = Controlled Drug Accountable Officer
Adverse events will be classed as green or red, depending on the degree of harm. Near misses, incidents that have caused no harm or minor harm will be classed as green. Moderate and major severity incidents, i.e. where significant harm has occurred, will be classed as red.
Community pharmacy contractors should acknowledge green incidents, complete an internal review and consider lessons to be learnt. Contractors must complete a full investigation into red incidents and respond to the health board with their findings within 28 days. If we do not receive a response to a letter relating to an incident classed as red within 28 days, we will send a further letter to the community pharmacy contractor, Area Manager and Superintendent Pharmacist.
Resources & Further Reading
- Healthcare Improvement Scotland Adverse events toolkit
- Scottish Government Organisational Duty of Candour: non-statutory guidance
- NES Turas Adverse event management
- NES Turas Duty of Candour
- General Pharmaceutical Council Keeping patients safe: being open and honest when things go wrong
.
Last updated EBR 10/06/25
