If you receive a prescription for an ULM, please consider if it is:
- Licensed (e.g. check BNF, or check MHRA products list)?
- Listed in Scottish Drug Tariff Part 7S or 7U?
- Available from UK NHS Manufacturing Site (e.g. Tayside)?
If YES to any above– authorisation not required
If NO- Please follow the ULM flowchart process below and complete the authorisation request form. These forms can be typed and should be emailed from your clinical mailbox to: ulmrequest@lanarkshire.scot.nhs.uk
Prescription Endorsement
Don’t forget specials/ULM items should be endorsed with the following:
- SP (Price of product e.g. if product was £45; endorse SP4500)
- HC 3000 (£30 fixed handling fee for Specials/ULM)
- PP (Any additional out of pocket charges e.g. if the product has a £15 delivery fee; endorse PP1500. If there was a £15 delivery fee & a £5 small handling fee; endorse PP2000)
ULM Community Pharmacy Flowchart
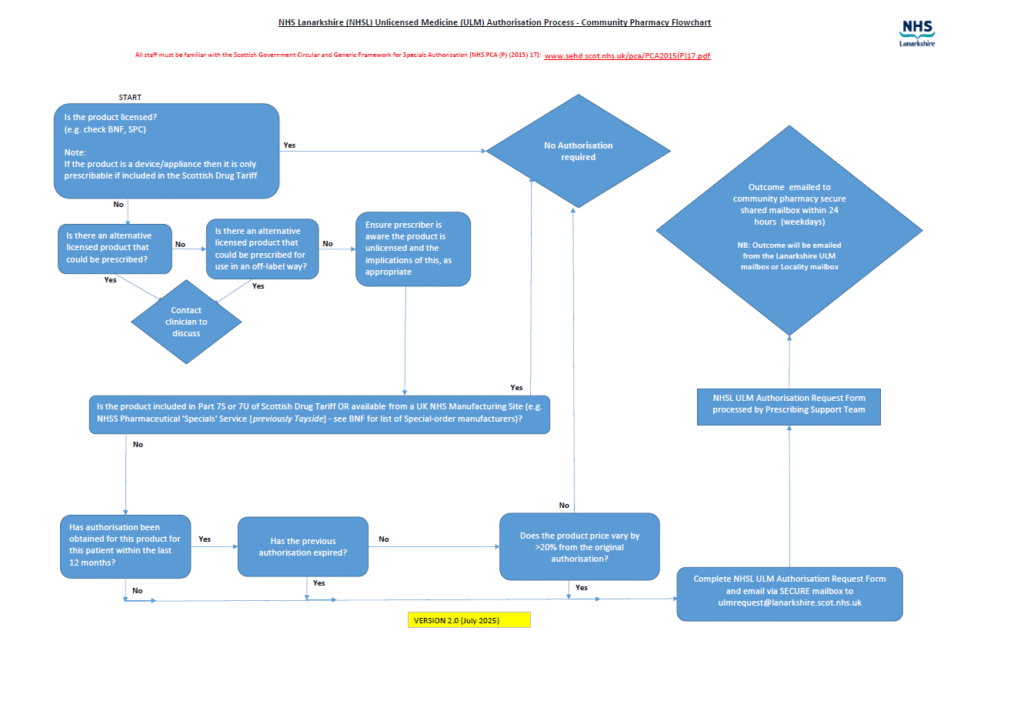
NHS LANARKSHIRE UNLICENCED MEDICINE PROCESS FLOWCHART (July 2025)
NHS LANARKSHIRE UNLICENCED MEDICINE AUTHORISATION FORM (July 2025)
All staff must be familiar with the Scottish Government Circular & Generic Framework for Specials Authorisation [NHS PCA (P) (2015) 17]
Outcomes
The board will authorise the product at the cost it has evidence for available at the time and provide the CP with the supplier details; the pharmacy may choose what supplier to source the product from, but will only be reimbursed at the stated cost.
This authorisation at the stated cost will remain for 12 months, unless communicated otherwise. Prices will be authorised up to 20% over this stated price during this 12 month period.
Clawbacks- The prescribing team will check and clawback any ULM costs that differ from the above. The pharmacies will be contacted and made aware of this.
