Process for authorisation and reauthorisation
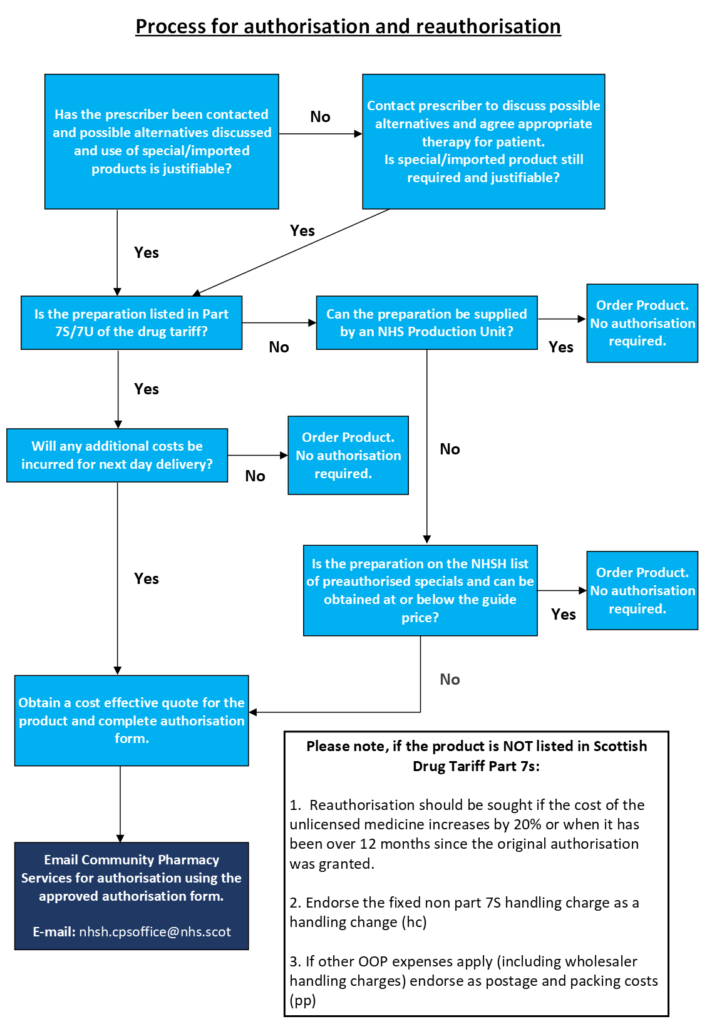
A downloadable version of the above flowchart can be found here.

Please ensure that invoices are submitted for unlicensed medicines, to PSD, with your monthly script submission.
Part 7s Liquid Preparations
For liquid preparations/product names – there is one price, the Part 7S price for all of the different liquid preps of a compound (e.g. solution, suspension, liquid, syrup etc). If there is a Part 7S price of a liquid preparation of a drug, then you will be paid that price whatever the liquid formulation. The Part 7S product name is also inclusive of any variation of excipients, e.g. sugar-free, colour-free, lactose –free etc. All will be priced at the Part 7S price.
Pre-Authorised List for Unlicensed Medicines (Updated November 2025)
Contact Numbers – Commonly Used Unlicensed Medicine Manufacturers
PSS Product price list July 2025
Medicines Shortages Information
PCA (P) (2015) 17 – Special Preparations and Imported Unlicensed Medicines
